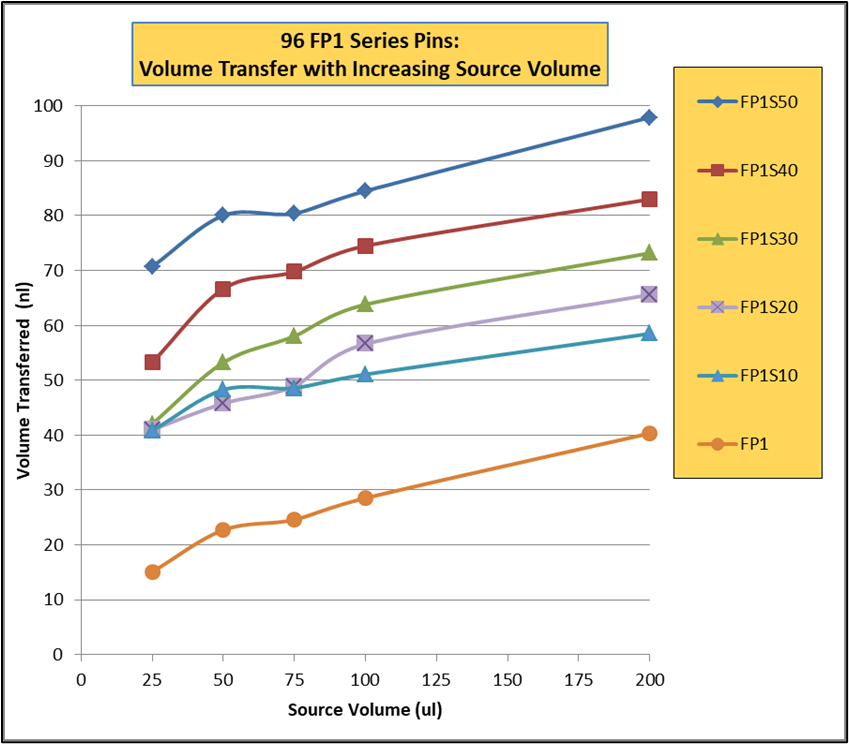
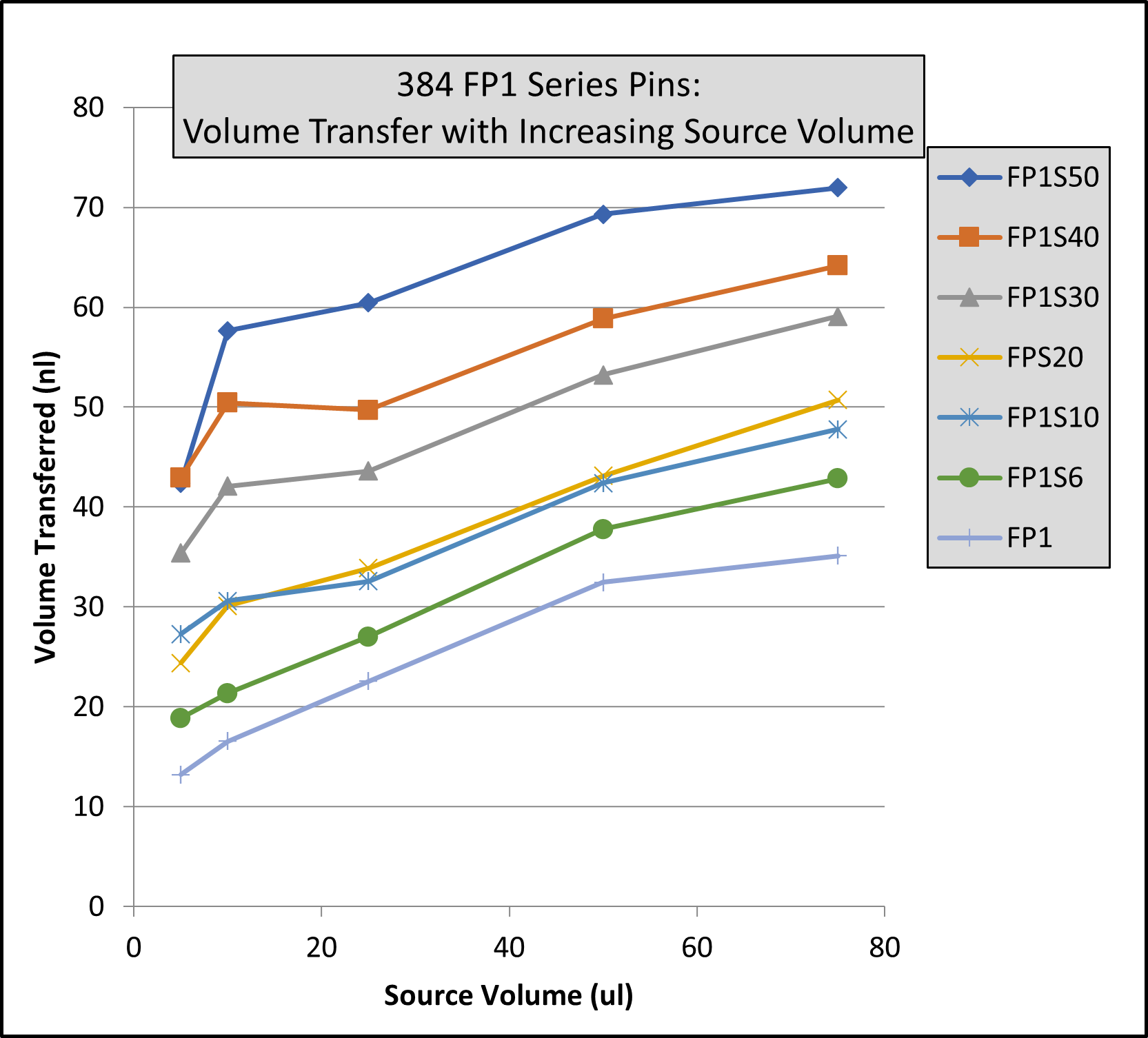
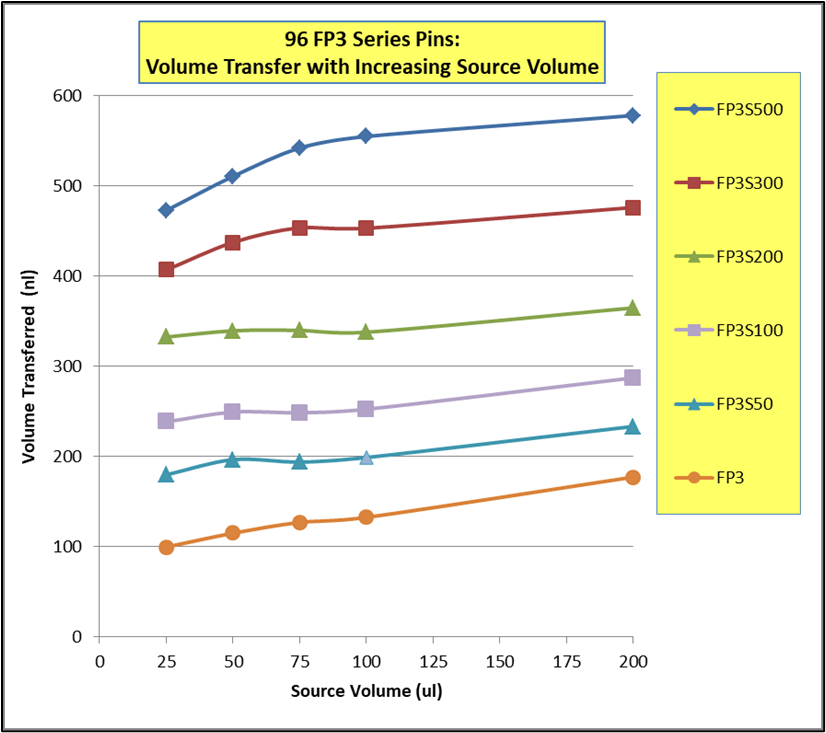
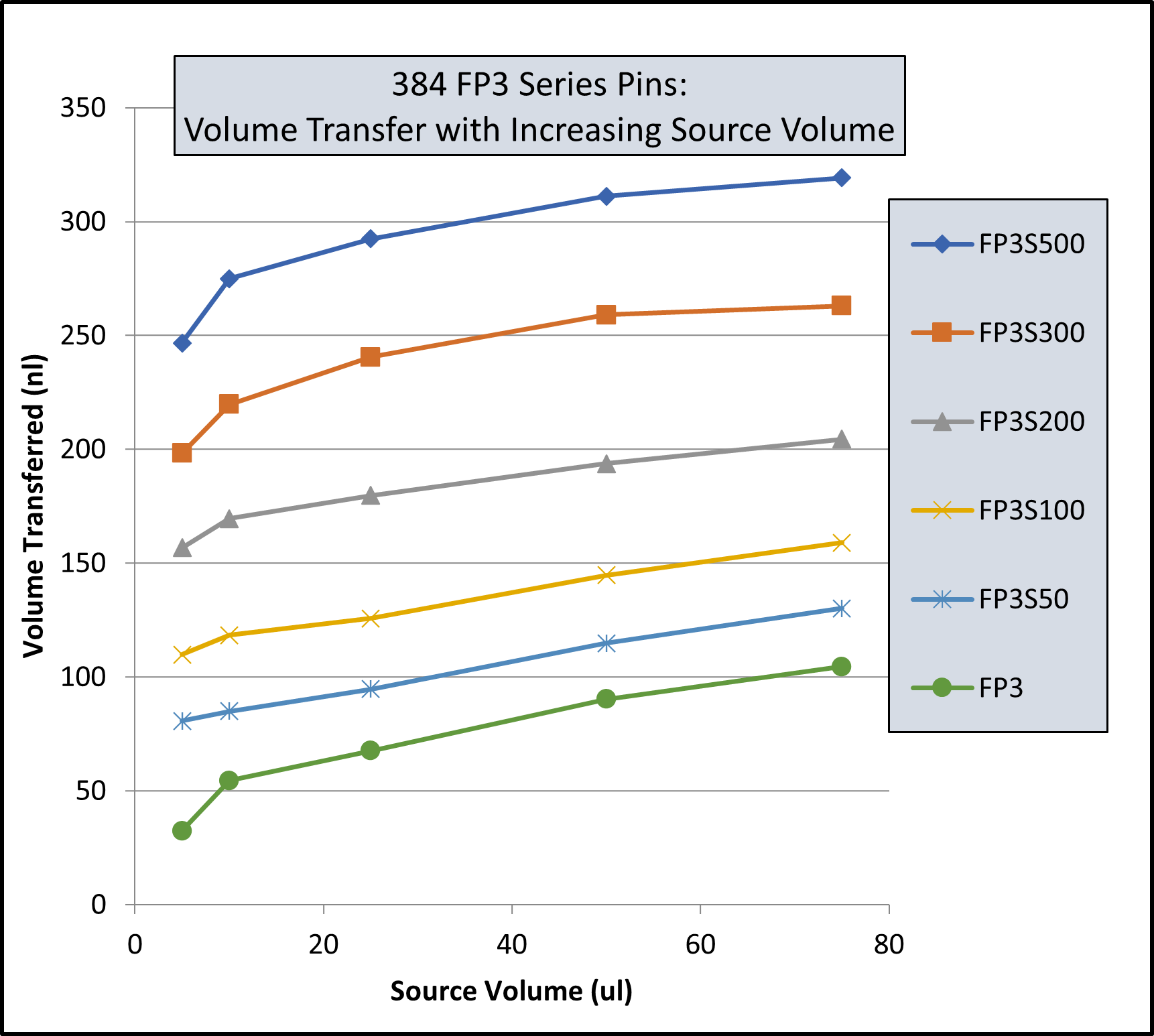
EJECTING STATION, for MagPin-R, VP 407AM-N1-R Cover Plate, and VP 407AM-N1-PCR, SLAS Footprint for Microplate Deck Positions, White Polypropylene
VP 407AM-N1-R-EJECT
EJECTING STATION, for MagPin-R
Efficient, Pipette-Free Transfer of Paramagnetic Beads
Designed to simplify and accelerate the transfer of paramagnetic beads between source and destination microplates, the VP 407AM-N1 eliminates the need for disposable pipette tips and multiple pipetting steps.
Ideal for applications like:
- DNA Sequencing Sample Prep: Next Generation Sequencing (NGS), 10X Sequencing
- Protein Sample Prep in Proteomic Research
- DNA/RNA Clean Up
- Magnetic Bead Assays
With its novel 96-MagPin® design, this tool enhances throughput results while minimizing bead loss and contamination. Our specially designed thin-walled 96-well PCR “cover plate” (VP 407AM-N-PCR) securely clips over the extractor, enabling efficient bead binding in one microplate and seamless release into another with minimal bead loss. Once captured, beads can be efficiently washed in one or more wash plates. Elution and transferring have never been easier; the cover plate can either be placed in a microplate with elution buffer or ejected into a destination microplate via stripper plate on the extractor, releasing the beads into solution.
Technical Advantages:
- Pipette-Free Operation: Reducing plastic waste and repetitive pipetting
- High Magnetic Strength: Ensures rapid bead capture and minimal loss
- Sterile Barrier Design: Prevents direct contact between beads and magnets
- Fast Wash & Elution: Optimized for high-throughput workflows
- Compatible with 96-well microplates: Seamlessly integrates into standard lab formats
Add It into Your Automation Today!
- For General Robots/Liquid Handlers: VP 407AM-N1-R with accompanying accessories
- For Robots with Grippers: VP 407AM-N-GRIP with accompanying accessories:
See It in Action:
Curious how real labs are using the VP 407AM-N1?
Check out what scientists are doing to enhance their research efforts on our blog.