STIRRER, Levitation, Linear Shuttle Motion, Stirs One Deep Well Microplate, with Smart Motor and a Worm Gear Drive, 110 Volts, 60 Hz
VP 707C
Levitation Stirrer, Shuttle Style
| Container Used | Deep Well Microplates |
|---|---|
| Mode of Use | Benchtop |
| Control Type | Manual Control |
| Power Supply Input | 110V |
| Frequency | 60 Hz |
| CE Compliant | Yes |
| Material | ABS |
| Magnet Strength | 42MGO |
Magnetic Levitation Stirrers
The V&P Scientific, Inc. Magnetic Levitation Stirrers (US patent #6,357,907; EU patent #1064988) were designed to stir the tall narrow columns of deep well 96 and Whatman Polyfiltronics deep well 384 microplates. As scientists have sought to increase the throughput of many processes they have turned to miniaturization and deep well microplates as a solution. However one of the problems of using deep well microplates is being able to adequately mix the well contents or resuspend particles in these tall narrow wells. Another problem was that of getting enough oxygen exchange through the relatively small surface area of the deep well microplates. We invented Magnetic Levitation Stirring as an efficient and inexpensive method to aerate and mix the contents of deep well microplates. The initial application was to resuspend human DNA which was amplified by replicating in bacteria in the bottom 384 deep well microplates. This levitation mixing facilitated the sequencing necessary for the Human Genome Project and was credited as being an “enabling technology” by Celera Genomics the company which was the first to successfully sequence 5 different humans. We were able to do this by simply raising and lowering stainless steel balls in all the wells by using a very strong horizontal magnetic field. This method produces a very vigorous stirring action that stimulates the growth of microorganisms, mixes with ease two or more liquids, and keeps particulates in suspension. Furthermore, if the media level in the wells is adjusted so the stir balls pass through the meniscus, aeration of microbial culture is increased and results in greater microbial yield as well as DNA and protein production.
The ability to stir extremely viscous solutions is a very useful characteristic of all the V&P Levitation Stirrers. Our Levitation Stirrers and Stir Balls are able to mix even solutions of 100,000 centistokes or 6.6 times more viscous than honey. A series of silicone viscosity standards were placed in the columns of a deep well microplate. The standard series viscosity was as follows: 100 cst, 1,000 cst, 5,000 cst, 10,000 cst, 12,500 cst, 30,000 cst, 60,000 cst and 100,000 cst.
For comparison 1 centistoke = 1.0760 x 10-5 sq. ft./sec.,
water = 1 cst
molasses ~ 2,500 cst
honey ~ 15,000 cst
Our VP 707B and VP 707C series Levitation stirrers can mix viscous solutions up to 30,000 cst while our VP 707E series Levitation stirrers are able to mix viscous solutions up to 100,000 cst because these stirrers use the magnet to also pull the stir balls down as well as to levitate them up. The VP 707B and VP 707C series Levitation Stirrers rely on gravity to pull the stir balls down.
Advantages of the System
- Thorough stirring of large numbers of samples
- Thorough stirring in tall narrow wells
- Will stir even viscous solutions – up to 100,000 centistokes
- No cross contamination – wells do not have to be sealed
- Simple to operate
Accessories
STIR ELEMENTS
The Levitation Stir Balls are made from corrosion-resistant stainless steel and come in a variety of sizes to accommodate all 96 and 384 deep well microplates. The Levitation Stir Balls are also provided encapsulated in parylene for combinatorial chemical reactions and other reactions that may be sensitive to metal ions. We can make any Levitation Stir Ball into a Sterile version contained in a Pyrex bottle. Although the cost of the balls is low enough to discard them after one use, they can be recovered, washed, demagnetized, and used over and over. Each of the Levitation Stir Balls is tested for magnetic energy and only those that pass our stringent testing are accepted.
DISPENSERS
Dispensers for loading the balls into the wells are also available for each ball size. These dispensers can quickly and accurately place sterile or non-sterile balls into the wells. One of the advantages of stainless steel balls over permanent magnets is that the stainless steel may be demagnetized so they don’t “clump” in the dispensers as the permanent magnets do.
REMOVAL SYSTEMS
Removing the balls from the wells after they are used can be done as easily and as sterilely as your assay conditions dictate. If your assay is completed and all the liquid has been removed, you can invert the microplate and magnetically collect the balls as they fall out. If the liquid is still in the wells and you want to remove the balls and leave the liquid behind just use the VP 770 Magnetic Plate System for stir ball extraction.
Scientists can apply this unique methodology to many different areas that require the mixing of liquids or suspending particulates in the tall narrow wells of deep well microplates. The most cost-effective is the manual Magnetic Levitation Stirrer VP 707. With as few as 10 back-and-forth strokes the contents of the wells are mixed. This Stirrer functions to levitate the balls by sliding the microplate through a horizontal magnetic field and then pulls the balls down by passing over a vertical magnetic field. The VP 707 is ideally suited to stir combinatorial chemical reactions or other reactions where continuous stirring is not required. The manual VP 707 has a footprint of only 20 cm wide by 64 cm long and 16 cm tall. This stirrer will stir one deep well microplate at a time at up to 100 lifts/minute.
All Levitation Stirrers are equipped with microplate “hold down shoes” designed to fit the Polyfiltronic/Whatman 384 deep well blocks (Uni Plate 400 – 400 ul volume) and ABgene’s 96 deep well blocks (2.2 ml volume) #AB-0661. Both these deep well blocks have a broad flat flange on the bottom and no skirt. This feature allows easy capture of the plate and holds it securely in a strong magnetic field. Hold-down shoes for other manufacture’s microplates will be done on a custom basis.
We also have microplate “hold down shoes” for Seahorse BioScience’s 4 ml 96 well blocks to fit on the VP 707C Linear Levitation Shuttle. We have also made custom “hold down shoes” for Seahorse’s 4 ml 96 well blocks that fit on the VP 707B Carousel.
V&P Scientific’s Unique and Patented Levitation Stirring Applications
- Resuspension of DNA in deep well microplates
- Assays to determine optimal biocide concentration in viscous solutions like lotions, toothpaste, mascara, paint, etc.
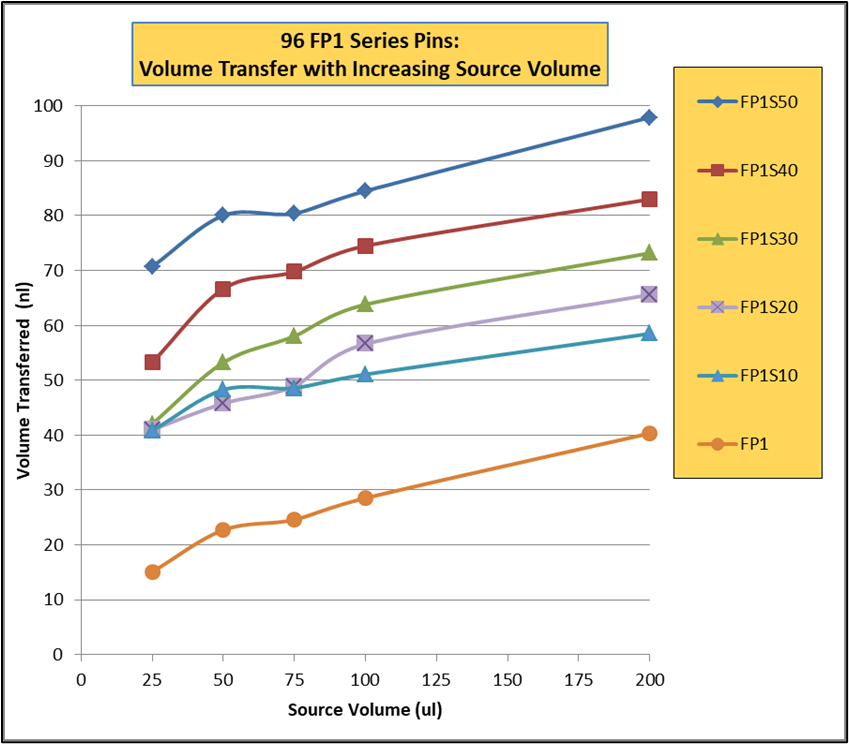
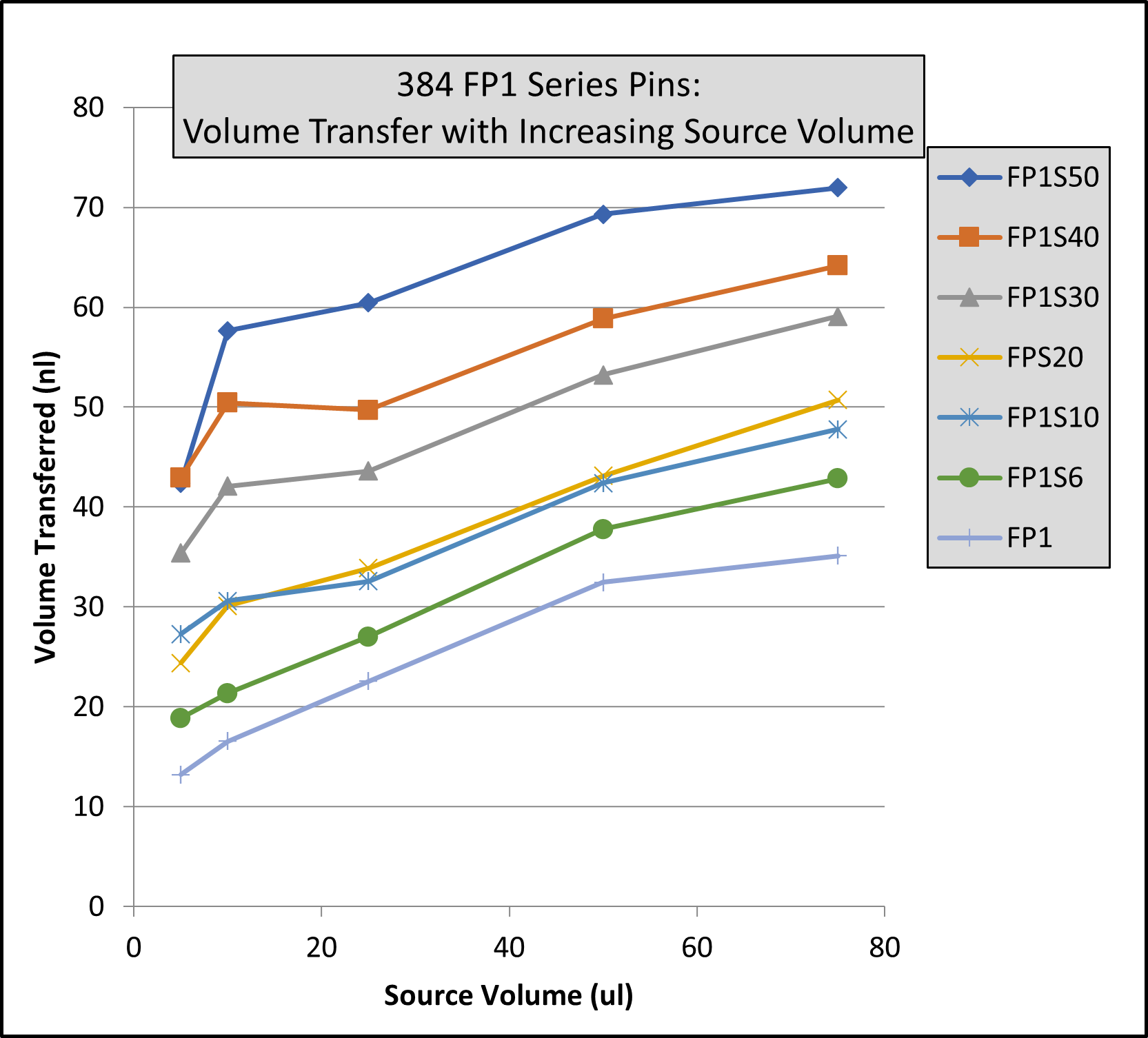
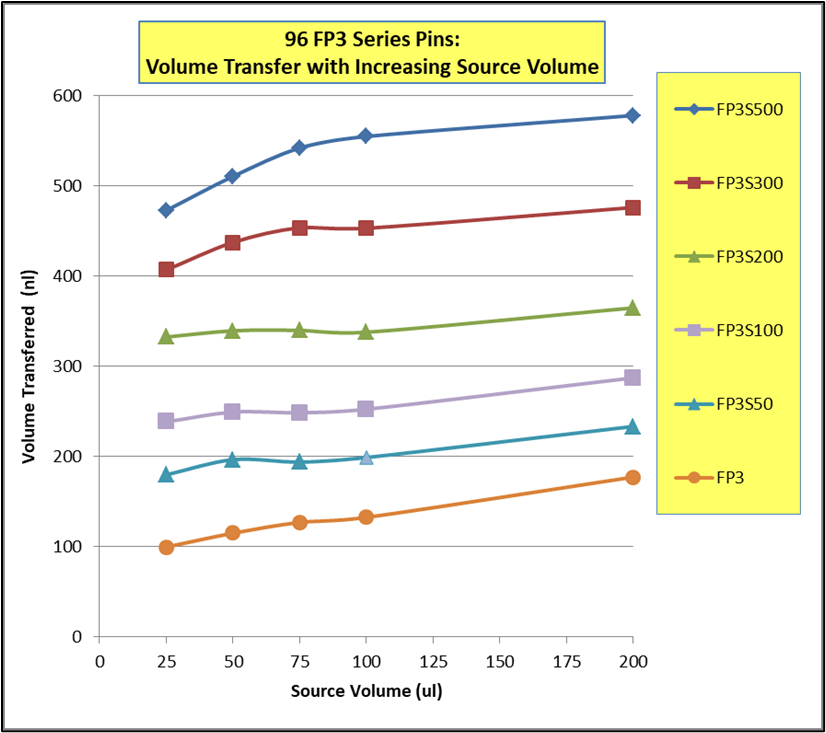
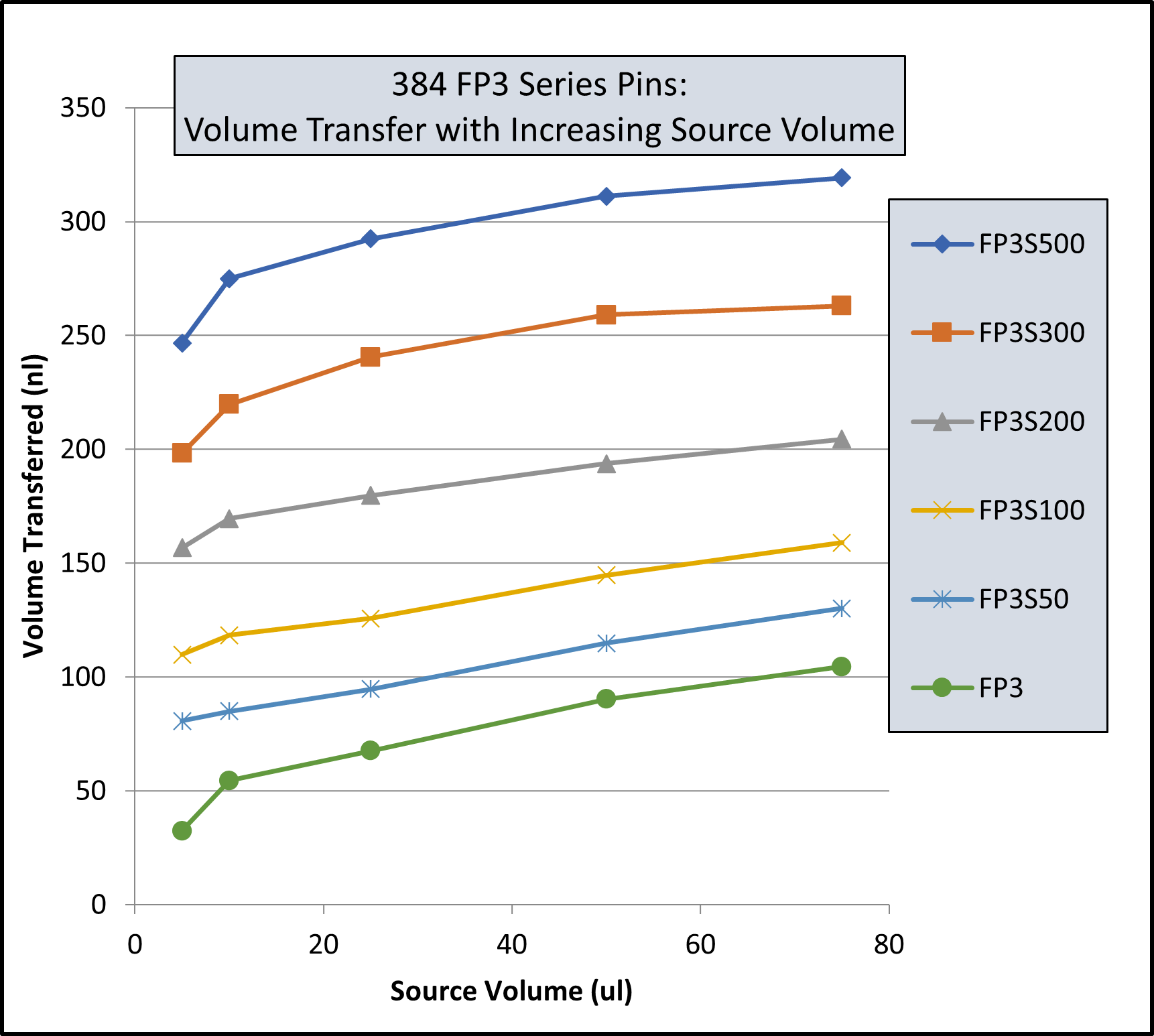
- Rapid and efficient stirring of 96 and 384 deep well microplates
- Thorough mixing of 2 or more viscous solutions
- Mix emulsions
- Keeps particulates in suspension
- Aeration of microbial cultures to increase DNA or protein yield
- Miniaturization of fermentations
Magnetic Field Strength versus Distance from the Alligator Magnetic Tumble Stirrer Deck
However, as a safety factor, we recommend that people with pacemakers keep at least 36″ or 91 cm from the Alligator Vertical Tumble Stirrers and the Vortex Lateral Tumble Stirrer. V&P does not make any claims in regard to the validity of the 5 gauss threshold for deactivating implanted medical devices. The 5 gauss magnetic field is a guideline to avoid risks associated with magnets and implanted medical devices such as pacemakers. For more information, please consult your physician. We try to position the magnet as close to the deck as possible given the constraints of the magnetic cylinders and deck design. Note the distance between the magnet surface and the surface of the deck varies for each magnetic tumble stirrer design and thus the gauss levels at the surface of the deck vary.